Cynthia Coulter, Catherine Castro
Immunalysis Corporation, Pomona, CA, U.S.A.
Objective
Tramadol, a CNS depressant and analgesic is used in the treatment of moderate to severe pain. The objective was to determine whether semi-quantitative data collected with HEIA has a strong correlation with routine immunoassay, ELISA.
Relevance
Since the main issues in clinical practice are the time and expense of sample processing, the development of a rapid semi-quantitative method for the screening of pain management drugs is necessary.
Methodology
® Oral fluid samples collected in the Immunalysis Quantisal® device were screened at a cut-off concentration of 50µg/L using ELISA and HEIA.
® Controls for ELISA and HEIA were diluted 1+3 with Quantisal® buffer to achieve neat oral fluid concentration.
® Positive samples were extracted using a previously published and validated solid phase extraction and analyzed using GC-MS. 1
Validation
Both assays: Calibration standards: 25, 50, 100, 200, 500µg/L
HEIA: Instrument: Olympus AU400e; Sample volume: 25µL; R1 and R2 volumes: 100µL Primary wavelength: 340nm; secondary wavelength: 410nm Measuring points 15 th -19 th rotation.
Inter-day precision was performed over 20 days with 2 replicates per day (N=40)
Coefficient of variation (CV): 150µg/L and 250µg/L - 0.65% and 0.64% respectively.
ELISA: Sample volume: 10µL. Intra-assay precision CV% for 150µg/L and 250µg/L - 5.35% and 4.05%, respectively Linearity r 2=0.9794 for ELISA semi-quantitative curve.
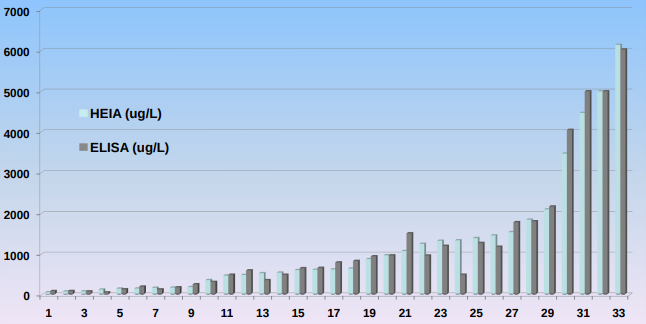
Results
® Thirty-three (33) oral fluid specimens previously found to be positive for tramadol using GC-MS were analyzed using HEIA and ELISA in the semiquantitative mode.
® Comparison of the data with the quantitative GC-MS data showed samples in the linear range of the assay to be within +/-20% of the confirmed concentration. Samples outside the upper limits of the curve and causing maximum absorbance readings were later diluted 1:10 or 1:100 and re-plated to fit within the standard curve range.
® Samples with screen values above the upper limits of the curve could be further diluted prior to extraction to eliminate the need for re-analysis, ultimately decreasing extraction costs and increasing throughput.
Conclusion
® Tramadol can be determined in oral fluid specimens using semi-quantitative screening modes on routine chemistry analysers or ELISA platforms.
® Both assays were rapid and simple to operate, showing a high degree of quantitative correlation with GC-MS.
Reference
Moore C, Rana S, Coulter C. Determination of meperidine, tramadol and oxycodone in human oral fluid using solid phase extraction and gas chromatography-mass spectrometry. J Chromatogr B. Biomed Applns 2007; 850: 370 - 375
SOFT, Oklahoma City, 2009
To view the full study and its data, visit this link.
Looking for equipment or accessories for your immunoassay and other related applications? Check out our products available on Block Scientific Store today!