Warren C. Rodrigues*, Catherine Castro, Philip Catbagan, James Soares and Guohong Wang
Immunalysis Corporation, Pomona, CA, USA
Abstract
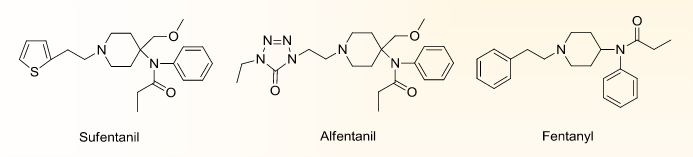
Sufentanil citrate marketed under the brand name Sufenta®, is a synthetic opioid analgesic drug, which is 5 to 10 times more potent than its analog, fentanyl. It is used for pain management over short periods of time. The sedative effects of sufentanil make it useful as an intravenous anesthetic. It is also being introduced in the form of patches of 20 and 100 ng. Moderate adult doses for major surgical procedures are about 2-8 µg/kg, then supplemental doses of 10-50 µg are given (1-2). The drug has an elimination half-life of 164 minutes in adults. The magnitude and duration of CNS and cardiovascular effects may be enhanced when it is administered to patients receiving barbiturates, tranquilizers, other opioids, general anesthetic or other CNS depressants.
Objective
Sufentanil is a Schedule II controlled substance. It can produce drug dependence of the morphine type and therefore has the potential for being abused. The goal of this study was to provide toxicologists with a novel ELISA screening method for detection of sufentanil in urine and blood.
Assay Method
- Sufentanil specific polyclonal antibodies were raised by immunization of rabbits with sufentanil antigen.
- Sufentanil IgG was then immobilized on microtiter plates for the assay.
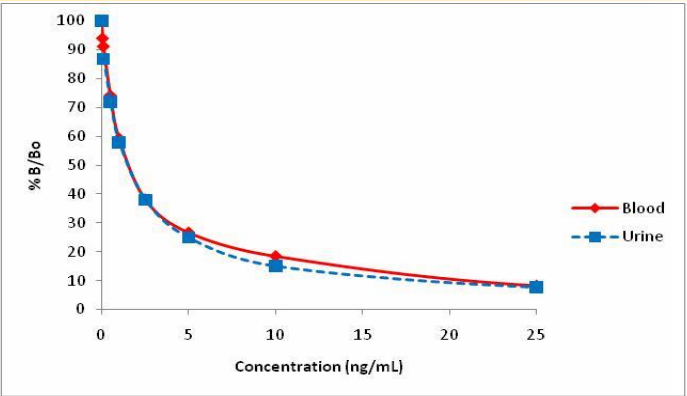
- Urine calibration curve was prepared at levels of 0.1, 0.5, 1, 2.5, 5, 10 and 25 ng/mL.
- Blood calibration curve was prepared at levels of 0.05, 0.1, 0.5, 1, 2.5, 5, 10 and 25 ng/mL in synthetic negative blood (after 1:10 dilution with PBS buffer).
- The sample sizes used were 10 µL for urine and 10 µL for blood (after 1:10 sample dilution).
- Sufentanil-HRP enzyme conjugate volume was 100 µL.
- The assay is colorimetric, TMB was used as substrate reagent and stopped with 1N HCl.
- Final absorbance was measured by a plate reader, at dual wavelengths of 450 and 650 nm.
Results
Recommended assay cutoffs: 5 ng/mL urine
0.5 ng/mL blood
Cross-reactivity
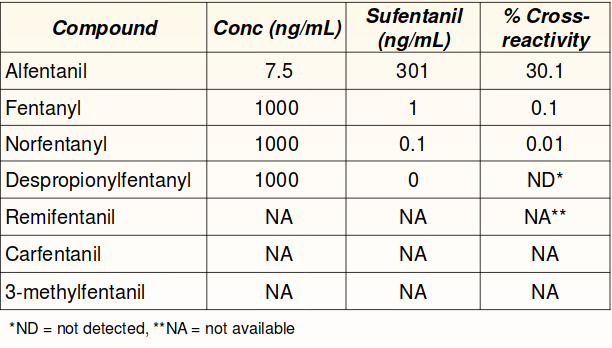
No cross-reactivity was detected with other common therapeutic drugs and drugs of abuse at 100,000 ng/mL.
Assay validation
Validation included intra-day (n=8) and inter-day (n=80) precision studies (<10% CV) for urine and blood at 0.5, 1, 2.5 and 5 ng/mL.
25 negative urine specimens were fortified with sufentanil at varying concentrations from 50 pg/mL to 500 ng/mL. No false positives or negatives were observed.
20 negative blood specimens were fortified with sufentanil at varying concentrations from 10 pg/mL to 10 ng/mL. No false positives or negatives were observed.
Summary
This paper describes a highly sensitive and specific ELISA method for detection of sufentanil in urine and blood. This assay would provide toxicologists with a reliable and efficient ELISA screen for monitoring sufentanil abuse using urine or blood.
References
- Disposition of Toxic Drugs and Chemicals in Main, 8th ed., Baselt R.C., Cravey R.H. 2009, 1455-1457.
- Evidence of addiction by anesthesiologists as documented by hair analysis, Kintz P., Villain M., Dumestre V., Cirimele V. J. Anal. Toxicol. 2005, 153, 81-84.
Soft Tiaft 2011
To view the full study and its data, visit this link.
Looking for a unit for your ELISA technique and other related applications? Check out our featured products below or visit Block Scientific Store today!