INTRODUCTION
Clopidogrel is an inactive thienopyridine prodrug which requires in vivo conversion in the liver to an active thiol metabolite which exerts its anti-platelet effect by forming an inactivating disulfide bond with the platelet P2Y12 adenosine diphosphate (ADP) receptor. Clopidogrel has been shown to effectively inhibit platelet aggregation and is at least as effective as aspirin in preventing cardiac events in patients with atherosclerosis.1
During the course of determining that cytochrome P450 CYP3A4 is the primary catalytic enzyme for the metabolic activation of clopidogrel in vivo, it was observed that the platelet anti-aggregatory effect after a loading dose of clopidogrel varied in healthy individuals.2 Parallel in vitro studies demonstrated that clopidogrel is metabolized by P450 CYP3A4, CYP3A5, and CYP2B6.3 CYP2B6 constitutes < 1% CYP in human hepatocytes: polymorphism in it's expression is unlikely to interfere with clopidogrel metabolism in vivo.3 CYP3A5 is primarily expressed in African Americans. Individuals with at least one CYP3A51 allele express large amounts of CYP3A5. Individuals with genetic polymorphism resulting in homozygous expression of CYP3A53, *5, or *7 do not have CYP3A5.4 Here we present preliminary data from three studies that support the hypothesis that differences in CYP3A4 activity largely account for the observed differences in the platelet inhibition response to clopidogrel.
METHODS
Study 1: Rifampin is a known inducer of CYP3A4 and would be expected to enhance the platelet inhibitory activity of clopidogrel.
* Human volunteers (n=10) received a maintenance dose of 75 mg/ day of clopidogrel for six days, followed by a washout period of 14 days, followed by four days of rifampin 500 mg BID, followed by both clopidogrel and rifampin for six days.
* Point-of-care platelet aggregation (Plateletworks®, Helena laboratories, Beaumont, TX and MICROS®, ABX Diagnostics, Irvine CA) was determined at days 0, 6, 20, 24, and 30.
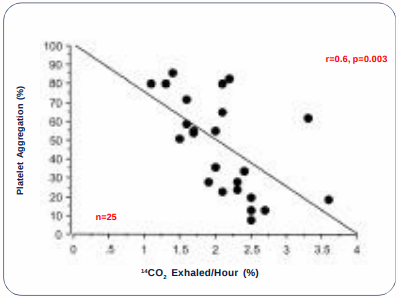
Study 2: A prospective study to investigate whether the variability in platelet aggregation by clopidogrel can be correlated with the metabolic activity of CYP3A4 measured by the erythromycin breath test (ERMBT, Metabolic Solutions, Inc., Nashua, NH).
* Human volunteers (n=25) received clopidogrel 450 mg.
* Point-of-care platelet aggregation was measured before and four hours after clopidogrel.
* ERMBT was measured before and two hours after clopidogrel.
* Linear regression analysis comparing platelet aggregation and ERMBT.
Study 3: A preliminary prospective study to determine whether CYP3A5 genetic polymorphism leads to variability in the response to clopidogrel.
* African American human volunteers (n=6) received clopidogrel 450 mg.
* Platelet function tests before and four hours after clopidogrel using:
- Point-of-care platelet aggregation
- Platelet-rich plasma aggregation (Chronolog Corp., Haverton, PA)
- Platelet surface P-selectin expression by flow cytometry (Dr. Alan D. Michelson, Center for Platelet Function Studies, Worcester, Massachusetts)
* Isolation of genomic DNA, amplification, and sequencing of CYP3A5 from whole blood component of each subject (Dr. Erin G. Schuetz, St. Jude Children's Research Hospital, Memphis, Tennessee).
RESULTS
STUDY 1: Percent Platelet Aggregation Response to Clopidogrel before and after Rifampin

STUDY 2: Percent Platelet Aggregation and ERMBT before Clopidogrel
STUDY 3: Genotypes of CYP3A5 and Platelet Activity Determined by Platelet Surface P-Selectin and Platelet Aggregation
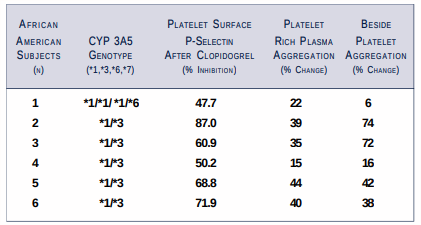
- CYP3A5*7 allelic polymorphism was not detected in the six subjects.
- These preliminary data demonstrate CYP3A5 polymorphism does not seem to predict metabolic activation of clopidogrel in vivo.
CONCLUSION
* Inter-individual variability exists in the response to clopidogrel.
* Preliminary data strongly support the hypothesis that the mechanism of variability in clopidogrel response is inter-individual differences in the expression of CYP3A4..
* These data warrant the awareness of potential drug-drug interaction with other CYP3A4 substrates.
* These data further suggest the need to monitor platelet activity during clopidogrel therapy to ensure pharmacologic efficacy.
REFERENCES
- CAPRIE Steering Committee. Lancet 1996;348:1329-39.
- Lau WC, et al. Circulation 2003;107:32-7.
- Clarke TA, et al. Drug Metab Dispos 2003;31:53-9.
- Kuehl P, et al. Nature Genetics 2001;27:383-91
2003 - auxier
Looking for point-of-care testing and other related applications? Browse through our featured products below.