Abstract
Benzodiazepines (after marijuana) are the most widely detected drugs in DUID cases. Oral fluid has become a useful biological testing matrix to detect recent drug usage, mainly due to observed collection and difficulty of adulteration of specimens. As roadside screening in some jurisdictions is moving to allow oral fluid, new assays are necessary for high volume screening. The sample volumes with oral fluid are limited and it typically contains lower concentrations of drugs. Also, the low saliva:plasma ratio (<1) for benzodiazepines, requires that new oral fluid assays be very sensitive and cross-reactive to the lower dosage drugs such as alprazolam, lorazepam, clonazepam, etc.
Oral Fluid Collection and Drug Stability
Using the Quantisal® oral fluid collection device, 1 mL of neat oral fluid is collected. The collection pad is immersed in a tube containing saliva extraction buffer (3 mL), capped and sent to a testing laboratory. To date, oral fluid analysis for benzodiazepines has been limited to an ELISA platform due to the lack of a homogeneous immunoassay (HEIA) and the need for a pretreatment step to reduce the viscosity problems associated with neat oral fluid. This collection method greatly simplifies the oral fluid testing. Benzodiazepines are relatively stable in the saliva extraction buffer. Based on GC-MS analysis, when various benzodiazepines were spiked into saliva extraction buffer and stored in the Quantisal® device at room temperature for 5 days, the recovery was 71.3 % for oxazepam, 92.1% for diazepam, 86.7% for alprazolam, 83.4% for lorazepam, 88.1% for clonazepam and 84.8% for temazepam.
Methods
This homogenous immunoassay uses a benzodiazepine antibody and is based on the competition of oxazepam labeled glucose-6-phosphate dehydrogenase (G6PDH) and the free drug in the oral fluid sample for a fixed amount of antibody binding sites. In the absence of free drug in the sample, the antibody binds the drug enzyme conjugate and enzyme activity is inhibited. This creates a dose response relationship between drug concentration in the oral fluid and enzyme activity. The enzyme activity is then measured spectrophotometrically at 340 nm by the conversion of NAD to NADH.
Assay Profile
Cutoff: 20 ng/mL; Detection limit: 1 ng/mL
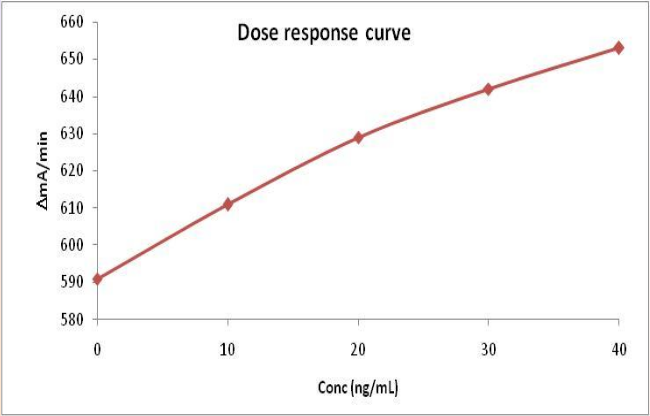
Assay Performance
Precision:
Inter-assay (5 days): 1-6 (S.D.); <1 (C.V.%)
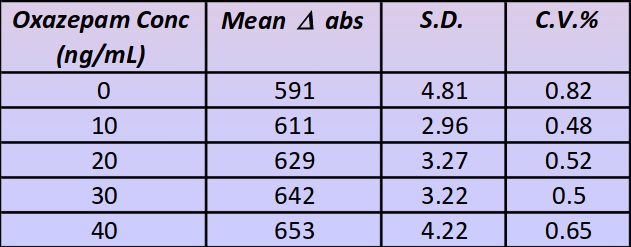
Intra-assay (n=5): shown in table below
Cross-reactivity
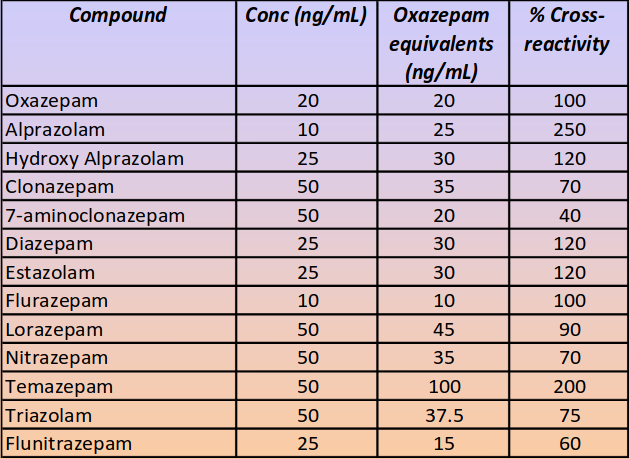
Non-related drugs and other interfering substances spiked in oral fluid, at a concentration of 10,000 ng/mL, showed no cross-reactivity and no false positive results in the assay.
Accuracy
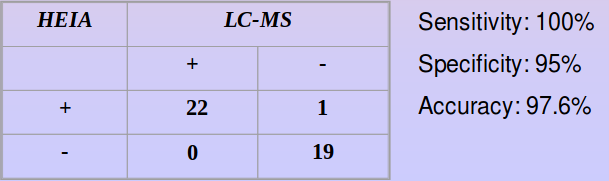
The one sample which tested positive by HEIA was screened at 18 ng/mL by GC-MS.
Summary
The homogeneous immunoassay is sensitive, specific and precise for the detection of a range of benzodiazepines in oral fluid, which are of significance in the toxicology field. The assay format is compatible with commercially available chemistry analyzers.
References
Saliva testing for drugs of abuse: Saliva as a diagnostic fluid, Cone, E.J. Annals NY Acad. Sci. 1993, 649, 91-127.
Methods for the measurement of Benzodiazepines in biological samples, Drummer, O. J. Chrom. B 1998, 713(1), 201-225.
Disclosure: Immunalysis Corporation manufactures and distributes the immunoassay described in this presentation
S.O.F.T. Annual Meeting, Phoenix, AZ, 2008
To view the full study and its data, visit this link.
Looking for equipment or accessories for your immunoassay and other related applications? Check out our products below.