Eleanor I Miller1 *, Hye-Ryun K Norris 1 , Douglas E Rollins 1 , Stephen T Tiffany 2 , Alpana Agrawal 3 , Christine M Moore 3 , Michael J Vincent 3 and Diana G Wilkins 1
1University of Utah, Center for Human Toxicology, 417 Wakara Way Suite 2111, Salt Lake City, Utah 84108 2 University at Buffalo SUNY, Department of Psychology, 228 Park
Hall, Buffalo, NY 14260 3 Immunalysis Corporation, 829 Towne Center Drive, Pomona, CA 91767
INTRODUCTION
Cigarette smoking is the leading cause of preventable death in the United States. The Centers for Disease Control and Prevention estimated that cigarette smoking produced 443,000 deaths each year in 2000-2004 and generated an estimated $157 billion in annual health-related economic losses.1 A recent national survey by the Substance Abuse and Mental Health Services Administration in 2007 estimated that 60 million people or 24 % of the United States population aged 12 or older had smoked cigarettes in the past month, highlighting the widespread use of nicotine.2
Although the prototypical cigarette smoker is characterized as someone who consistently smokes at least 1 pack of cigarettes or more per day, a large number of current smokers neither smoke daily nor consume 1 pack of cigarettes on days on which they do smoke. These "low-level" irregular smokers pose an increased health risk as a consequence of the inhalation of tobacco smoke, including increased rates of coronary heart disease, myocardial infarction and lung cancer compared to nonsmokers.3,4 The transition from "low-level smoker" to nicotine dependence has been documented however little is known about the processes that underlie changes in smoking behavior from the first use of a cigarette, subsequent regular use and eventual addiction.
The relationship between cigarette smoking, exposure to ETS and serious health conditions has resulted in the need for analytical methods for the determination of nicotine and metabolites in biological samples. This current research is focused on the investigation of biomarkers of nicotine use in the oral fluid of "low-level" smokers.
AIM
There were several study objectives: (1) To develop and validate a sensitive and specific liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for the simultaneous quantification of nicotine-N-β-glucuronide (NIC GLUC), cotinine-N-oxide (CNO), trans-3-hydroxycotinine (3-HC), norcotinine (NCOT), trans-nicotine-1'-N-oxide (NNO), cotinine (COT), nornicotine (NNIC), nicotine (NIC), anatabine (AT), anabasine (AB) and cotinine-N-β-glucuronide (COT GLUC) in human oral fluid collected as part of a clinical study investigating biomarkers of low-level smoking; and (2) To obtain preliminary data on the use of the COT microplate enzyme-linked immunosorbent assay (ELISA) as a screening tool in the analysis of oral fluid collected from low-level smokers.
HUMAN SUBJECTS
Clinical samples were collected as part of an Institutional Review Board approved study (IRB #21414, University of Utah). One milliliter of oral fluid was obtained from three non-smoker human subjects using the Quantisal® collection device (Immunalysis Corporation, CA) prior to the application of a 7-mg transdermal nicotine patch (Novartis ®, Basel, Switzerland) and then at 0.5 h and 0.75 h following patch removal. The patch was worn for four hours to mimic the plasma nicotine concentration observed after smoking 1 cigarette.5 Anatabine and anabasine were included in the method to facilitate monitoring of unauthorized tobacco use by participants during the study since these two alkaloids are derived from the leaves of the tobacco plant.
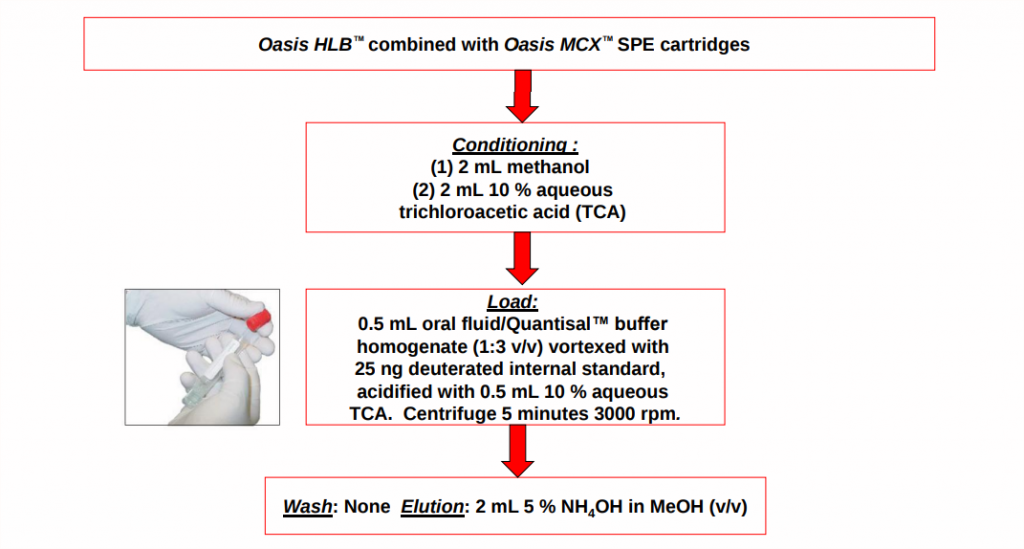
SPE METHOD
ELISA METHOD
20 μL of oral fluid/Quantisal® buffer homogenate (1:3 v/v) was added to the COT microplate ELISA wells in duplicate using a Miniprep 75 automatic pipettor system (Tecan Group Ltd, Männedorf, Switzerland). 100 μL of COT enzyme conjugate reagent was subsequently added and the plate left in the dark at room temperature for an incubation period of 1 h. Following incubation, the microplate wells were washed with deionized water (6 x 350 μL) in order to remove any unbound sample or residual enzyme conjugate reagent that may be left in the wells. 100 μL of 3,3',5,5'-tetramethylbenzidine (TMB) substrate reagent was then added to the wells and the plate left to incubate in the dark at room temperature for an additional 30 minutes. The reaction was stopped after this time by adding 100 μL of 1 N hydrochloric acid (stop reagent). The well contents turned from blue to yellow after addition of the acid to allow detection of the TMB chromophore at a wavelength of 450 nm using a Sunrise® Micro-plate Reader (Tecan Group Ltd, Männedorf, Switzerland).
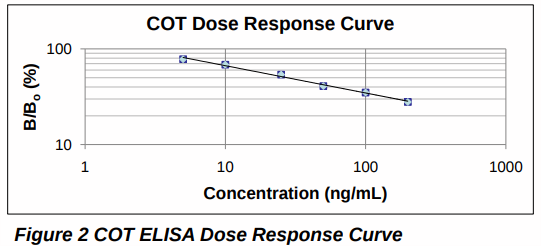
The ELISA dose response curve was generated by comparing the absorbance value for fortified analyte-free oral fluid/Quantisal® buffer homogenate (B) with analyte-free oral fluid/ Quantisal® buffer homogenate (B0 ) over the range 5-100 ng/mL COT. Intra-day imprecision within the same batch (n=8) and inter-day imprecision for 8 different replicates analyzed in 10 separate batches (n=80). Cross-reactivity (n=2) was calculated for all analytes in the LC-MS/MS method at a concentration of 50 ng/mL relative to the COT dose response curve. A cut-off concentration of 10 ng/mL COT was selected for the screening of clinical samples.
Chromatographic separation: ® Discovery ® HS F5 LC column (100 mm x 4.6 mm, 3 µm, Supelco ®, MO) ®Mobile phase 10 mM ammonium acetate with 0.001 % formic acid (pH 4.97) (A), and methanol (B) at a flow rate of 0.6 mL/min. ® Initial mobile phase conditions were: 15 % B increased linearly to 76 % B after 11 minutes; then decreased back to 15 % B after 11.6 minutes and held for 3.4 minutes to re-equilibrate the LC column (total run time 15 minutes).
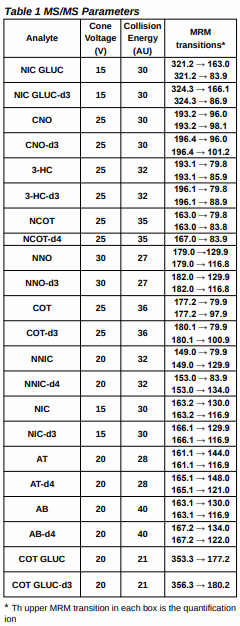
Mass spectrometric analysis: ® Quattro Premier XE® triple quadrupole mass spectrometer (Waters ® Corporation, MA) with MassLynx® v 4.1 software ® Operated in electrospray positive mode using multiple reaction monitoring (MRM) data acquisition. ® Two MRM transitions monitored for each analyte and deuterated internal standard with the exception of COT GLUC, COT GLUC-d3 and NCOT-d4 (which produced only 1 fragment ion). Cone voltages, collision energies and selected MRM transitions are given in Table 1.
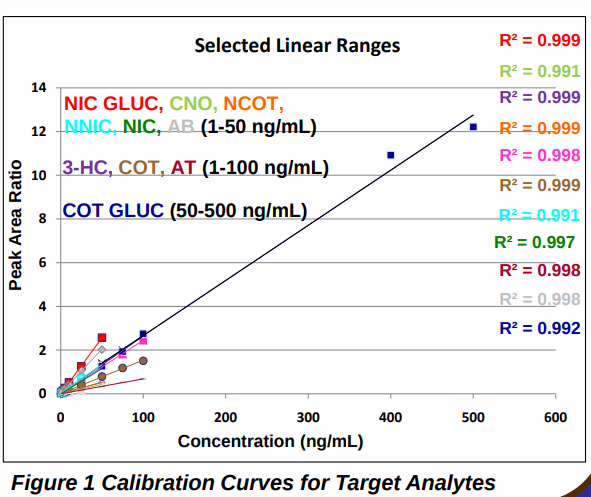
LC-MS/MS method validation ®Assessment of specificity, linearity over the expected concentration range for each analyte (Figure 1), limit of quantification (LOQ), extraction recovery, intra-day and inter-day imprecision. 6
LC-MS/MS and ELISA METHOD VALIDATION RESULTS
LC-MS/MS:
Method specificity was assessed by the triplicate analysis of blank human oral fluid collected from six different nicotine abstinent individuals using the Quantisal® collection device. There were peaks for the quantification ion transition for NIC GLUC and NIC (signal-to-noise (S/N) >10), and for COT and NNIC (S/N >5), however the peak area ratios of analyte quantification ion to deuterated internal standard quantification ion were at least 20 % lower than the peak area ratio for the LOQ and there were no peaks present for the second MRM transition. Calibration curves (Figure 1) were linear over the concentration range selected for each analyte with coefficients of determination (R2 ) of > 0.99. The LOQ for all analytes in oral fluid/Quantisal® buffer homogenate was 1 ng/mL with the exception of COT GLUC, which was 50 ng/mL; intra-day (n=5) and inter-day (n=15) imprecision were ≤ 15 % and ≤ 18 % respectively. The % total extraction recovery was calculated at a low, medium and high concentration for each analyte by comparing the peak area ratio of the quantification ion to deuterated internal standard quantification ion for extracted samples, with unextracted samples. Mean recoveries (n=5) were concentration dependent and ranged from 80-119 %.
ELISA:
The ELISA dose response curve was linear, for COT, with an R2 value of > 0.99 over the range 5-100 ng/mL (Figure 2). Intra-day and inter-day imprecision were < 3 % for the chosen assay cut-off concentration of 10 ng/mL COT. Analytes which were targeted in the LC-MS/MS method, were tested for cross-reactivity with the COT microplate ELISA and did not cross-react, with the exception of 3-HC which cross-reacted by 80 % at a COT concentration of 50 ng/mL.
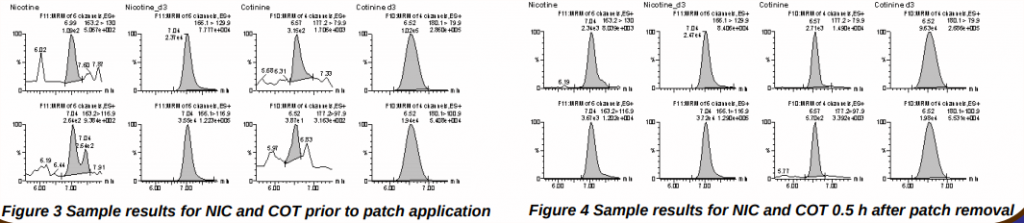
Figures 3 and 4 show chromatograms of a study participant's oral fluid sample collected before application of the nicotine patch (Figure 3) and an oral fluid sample collected 0.5 h after removal of the patch (Figure 4). None of the target analytes were detected in (Figure 3) whereas 40.6 ng/mL NIC and 11.9 ng/mL COT were detected in (Figure 4).
CLINICAL SAMPLE RESULTS
A comparison of COT data was made for LC-MS/MS and ELISA methods. Baseline oral fluid samples collected were negative by both methods. Samples collected 0.5 h and 0.75 h after patch removal contained 5.7-24.5 ng/mL COT and 6.6-31.2 ng/mL NIC; and 7.8-26.3 ng/mL COT and 12.9-36.8 ng/mL NIC by LC-MS/MS respectively. Consistent with the 10 ng/mL ELISA cut-off, and as predicted by LC-MS/MS data, two of the three participants had ELISA-positive samples at the two collection points following transdermal NIC delivery.
CONCLUSION
A sensitive and specific LC-MS/MS and ELISA method have been successfully applied for the quantification and detection of COT in oral fluid samples collected after removal of a 7-mg transdermal nicotine patch which had been worn for four hours as part of a clinical study investigating nicotine biomarkers in low-level smokers.
ACKNOWLEDGEMENTS
This research was supported by the National Cancer Institute (Grant # R01 CA 12040412). The authors would like to thank the Center for Clinical and Translational Science (CCTS) at the University of Utah Health Sciences Center for financial support (#ULIRR025764) and the CCTS staff for help with the study. Thanks also to the Immunalysis Corporation for the generous travel grant donation for Dr Eleanor I Miller.
REFERENCES
1 Centers for Disease Control (2008). Smoking – Attributable mortality, years of potential life lost, and productivity losses, United States, 2000- 2004
2 Substance Abuse and Mental Health Services Administration (2008). Results from the 2007 National Survey on drug use and health (Office of Applied Studies, NSDUH H-34, DHHS Publication No SMA08-4343), Rockville, MD
3 Rosengren A., Wilhelmsen L., Wedel H. Coronary heart disease, cancer and mortality in male middle-aged light smokers. Journal of Internal Medicine 231, 357-362
4 National Cancer Institute (1998). Cigars: Health effects and trends. Smoking and Tobacco Control. Monograph No. 9
5 Patterson F., Benowitz, N.L., Shields P., Kaufmann V., Jepson C., Wileyto P., Kucharski S., Lerman C. Cancer Epidem. Biomar. 12: 468-471 (2003)
6 Miller EI., Kim H.K., Rollins D.E., Tiffany S.T., Wilkins D.G. SOFT poster 2009
To view the full study and its data, visit this link.
Looking for equipment or accessories from Immunalysis? Visit Block Scientific Store today!