Guohong Wang*, Kim Huynh, Rehka Barhate, Warren Rodrigues, Christine Moore, Michael Vincent, and James Soares Immunalysis Corporation, Pomona, CA USA
Introduction and Objective
The objective of this project was to develop and validate a high throughput homogeneous enzyme immunoassay (HEIA) for the rapid detection of ethyl glucuronide in human urine.
Ethyl Glucuronide (EtG) is a minor metabolite of ethanol. Ethanol is primarily used as a social drug; however it can be found in mouthwashes, medical liquids, and manufactured solvent and gasoline additives, therefore it is not an ideal biomarker for alcohol consumption. Ethanol metabolizes about 95% of its dose and the remaining 5% is unchanged in breath, urine, sweat and feces. EtG is used as a biomarker for ethanol intake over its major metabolite, acetaldehyde due to its slow elimination in urine and specificity for ethanol. EtG can be detected up to 130 hours at a cutoff of 500 ng/mL(1). For this reason, it is valuable to develop and validate a homogeneous immunoassay (HEIA) to detect EtG in urine.
Advantages
- Ready to use reagents suitable for high throughput instruments
- Assay working range : 0 to 1500ng/ml with duel cutoff concentrations at 500ng/ml and 1000ng/ml
- Specific assay with accuracy >95% based on 54 urine specimens
Results and Discussion
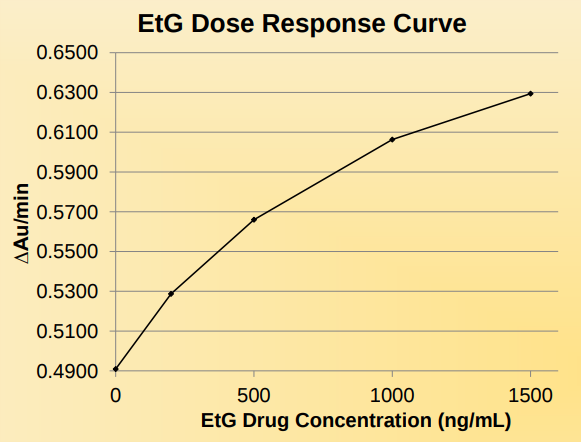
Precision: Daily Calibration Required
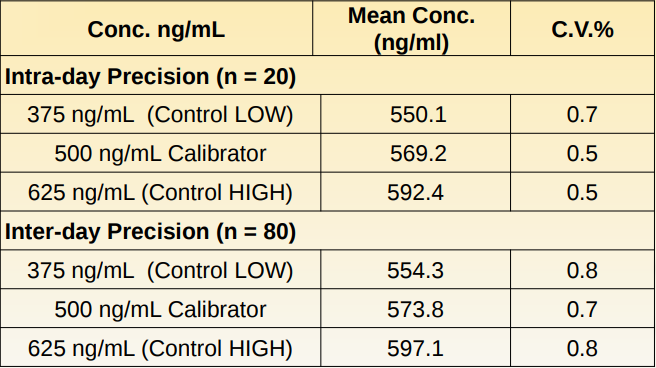
The precision was determined by assaying calibrators and controls in synthetic urine for 4 days, 2 runs per day in replicates of 10 (N=80). The results are summarized below.
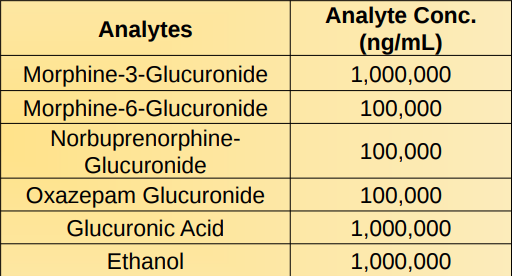
Cross Reactivity:
Structurally related compounds that are potentially found in urine were tested using the 500 ng/mL cutoff calibrator. All these compounds produced a negative value at the concentration listed on table below.
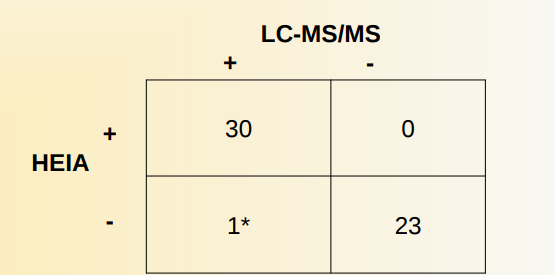
Authentic specimens:
- 54 urine specimens previously confirmed by an outside laboratory by LC-MS/MS were analyzed with this Immunalysis Etg EIA assay
- Cutoff concentration: 500 ng/mL for both EIA and confirmation method
- 23 specimens were negative by both methods
- 30 specimens were positive by both methods
- The sensitivity, specificity and accuracy were 97%, 100%, and 98%, respectively
- Negative result: absorbance rate reading just below cutoff
- Confirmation = EtG 663 ng/mL, EtS 339 ng/mL.
Summary
A high throughput HEIA has been developed for the detection of ethyl glucuronide in human urine which correlates well with LC-MS/MS.
References
- G.Reisfield, B.Goldberger, B.Crews, A. Pesce, G. Wilson, S. Teitelbaum, and R. Bertholf. Ethyl glucuronide, ethyl sulfate, and ethanol in urine after sustained exposure to an ethanol-based hand sanitizer. J. Anal. Toxicol. 35: 85-91 (2011)
SOFT, Boston, MA 2012
To view the full study and its data, visit this link.
Looking for equipment or accessories for your immunoassay and other related applications?? Check out our products below.