Introduction and Objective
The objective of this project was to validate a new high throughput homogeneous enzyme immunoassay (HEIA) for the rapid detection of tapentadol in human urine. Tapentadol is a new and potent opioid analgesic that was approved by the FDA in 2008 for
Advantages
- Ready to use reagents suitable for high throughput instruments
- Targeting the parent drug and O-glucuronide metabolite
- Highly sensitive and specific with low
cross reactivity with amphetamines and tramadol
Results and Discussion
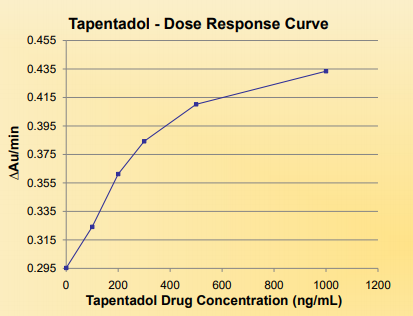
Precision: Daily Calibration Required
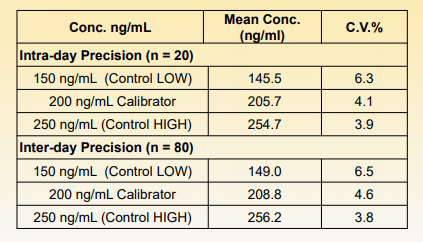
The precision was determined by assaying calibrators and controls for 4 days, 2 runs per day in replicates of 10 (N=80). The results are summarized in
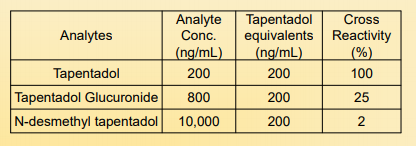
Cross Reactivity:
The table below represents related drugs that have cross reactivity with the EIA assay.
The table below represents unrelated drugs that could yield screening positive results at high concentrations
Authentic specimens:
A total of 191 urine specimens previously confirmed with LC-MS/MS by external reference laboratories were analyzed using the HEIA assay at a cut off of 200ng/mL; the confirmation cut off was 50ng/mL. Due to the

The sensitivity, specificity, and accuracy were calculated to be 97%, 98%, and 98
Summary
A high throughput HEIA has been developed for the detection of tapentadol and one of its major metabolites in human urine which correlates well with LC-MS/MS. This is the first report of a homogeneous immunoassay for tapentadol
References
- C. Coulter, M. Taruc, J. Tuyay C. Moore. Determination of tapentadol and its metabolite N-
desmethyltapentadol in urine and oral Fluid using liquid chromatography with tandem mass spectral detection. J. Anal. Toxicol. 34: 458-463 (2010). - J.A. Bourland, A.A. Collins, S.A. Chester S.Ramachandran, R.C.Backer. Determination of tapentadol (Nucynta®) and N-
desmethyltapentadol in authentic urine specimens by ultra-performance liquid chromatography-tandem mass spectrometry. J. Anal. Toxicol. 34: 450-457(2010).
SOFT/TIAFT San Francisco, CA 2011
To view the full study and its data, visit this link.
Looking for a unit for your immunoassay applications? Check out our featured products below.