Abstract
Benzylpiperazine (BZP) is a psychoactive stimulant belonging to the piperazine class of designer "party" drugs (1). The aryl piperazine compounds are selective for certain serotonin receptors and produce euphoric effects similar to methamphetamine and ecstasy. Law enforcement agencies in the US have reported finding BZP along with 3-trifluoromethylphenylpiperazine (3- TFMPP) and 3-chlorophenylpiperazine (3-CPP) in 33% of seized ecstasy tablets (2). Capsules containing up to 250 mg BZP dihydrochloride, sold under the name A2 have appeared on internet websites (3). This prompted the action by the DEA in 2004 to declare BZP as a schedule I drug substance.
Objectives
Toxicology labs currently exploit the small cross-reactivity with amphetamine and MDMA homogeneous assays or use expensive GC-MS and LC-MS/MS methods for detection of BZP. The goal of this study was to provide toxicologists with a highly specific ELISA BZP screening method for urine and blood.
Assay method
- BZP specific polyclonal antibodies were raised through immunization of rabbits with BZP antigen.
- IgG was further immobilized on microtiter plates for the assay.
- Calibration curves were prepared at levels of 5, 10, 25, 50, 100 and 250 ng/mL in synthetic negative urine and synthetic negative blood.
- The sample sizes used were 10 µL for urine (after 1:20 sample dilution) and 20 µL for blood (after 1:10 sample dilution).
- BZP-HRP enzyme conjugate volume was 100 µL. 6. The assay is colorimetric, TMB was used as substrate reagent and stopped with 1N HCl. 7. Final absorbance was measured by a plate reader, at dual wavelengths of 450 and 650 nm.
Results and Discussion
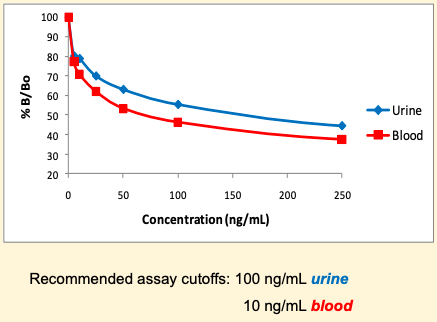
The ELISA assay employs competitive binding between the enzyme conjugate and free analyte in the sample for a fixed amount of antibody binding sites, which is proportional to their concentration in the mixture.
Cross-reactivity
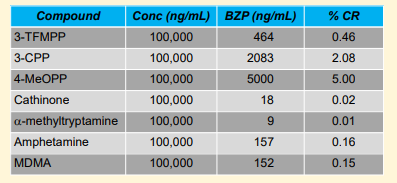
No cross-reactivity was detected with other common therapeutic drugs and drugs of abuse at 100,000 ng/m
Assay validation
Validation included intra-day (n=8) and inter-day (n=80) precision studies (<10% CV) at 10, 25, 50 and 100 ng/mL Four matched pairs of postmortem urine and blood specimens obtained from the LA County Coroners Lab were screened. 2 pairs of urine and blood screened positive, 1 pair was negative for both matrices and the last pair screened positive for blood, but negative for urine. These results matched the GC-MS screen provided, except for the last urine, which gave a false negative result in the ELISA, due to being close to cutoff.
Summary
We have developed a highly sensitive and specific method for detection of BZP in urine as well as blood. This assay would provide a useful ELISA method to toxicologists attempting to screen for BZP usage, as Compound Conc (ng/mL) BZP well as for postmortem drug screening.
References
- Disposition of Toxic Drugs and Chemicals in Man, 8th ed., Baselt R.C., Cravey R.H. 2009, 155-156.
- Detection of 1-benzylpiperazine and 1-(3-trifluoromethylphenyl) piperazine in urine analysis specimens using GC-MS and LCESI-MS, Vorce S.P., Holler J.M., Levine B., Past M.R. J. Anal. Toxicol. 2008, 32, 444-450.
- Piperazine like compounds: a new group of designer drugs of abuse on the European market, de Boer D., Bosman I.J., Hidvégi E., Manzoni C., Benko A.A., dos Reys L.J.A.L., Maes R.A.A. Forensic Sci. Int. 2001, 121, 47-56.
SOFT/TIAFT San Francisco, CA 2011.
To view the full study and its data, visit this link.
Looking for a unit for your ELISA technique and other related applications? Check out our featured products below.