Abstract
Diphenhydramine (DPH) is an over-the-counter first generation antihistamine, commonly marketed under the trade name Benadryl®. Even though it is one of the oldest antihistamines, it is more effective and fast acting than some of the latest prescription allergy medications. It belongs to the ethanolamine class of drugs and exerts its action by blocking the effects of histamine at the H1 receptor sites (1-2). The dosage for adults is in the range of 25-50 mg every 4-6 hours. The half- life of DPH is 2-9 hours. Although most of the dose (about 64%) is excreted as metabolites, the parent drug is also found to be present in the urine and blood. The structures of some of the various antihistamines are shown below.
Objective
DPH produces marked drowsiness in patients taking the drug, which makes driving and taking the medication dangerous. This leads to driving accidents and fatalities. The goal of this study was to develop an ELISA method to allow toxicologists to screen urine and blood DUID specimens for the presence of DPH.
Advantages
Diphenhydramine ELISA provides the following advantages:
* Quick screen for antihistamines in urine and blood
* Can be used as part of a DUID toxicology panel
* Highly sensitive immunoassay
Assay method
The assay employs competitive binding between DPH - enzyme conjugate and free drug in the sample, for a fixed amount of antibody binding sites. A complete description of the assay method is described in the Immunalysis DPH ELISA kit insert.
Assay parameters and performance
Sample size: 10 µL
Recommended urine cut-off: 10 ng/mL (1:20 sample dilution)
Recommended blood cut-off: 1 ng/mL (1:10 sample dilution)
Precision: (run at 5, 10, 25, 50 ng/mL)
Intra-assay (n=8): ELISA < 10% CV
Inter-assay (n=80): ELISA < 10% CV
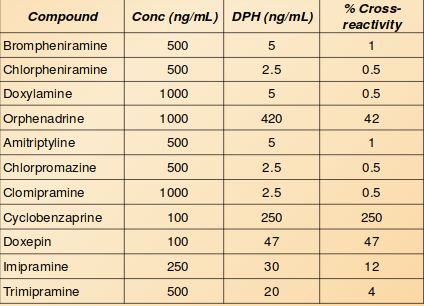
Cross-reactivity
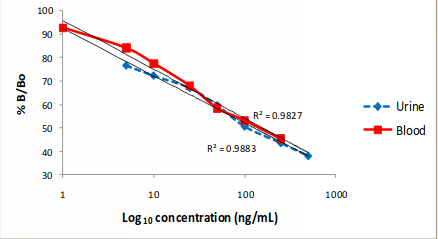
Assay Validation
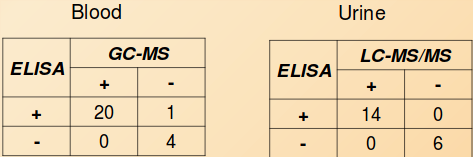
The one blood sample which tested borderline positive by ELISA, was found to be negative for DPH, but positive for cyclobenzaprine when confirmed by GC-MS using a LOQ of 100 ng/mL
Summary
The described method is sensitive and precise for the detection of diphenhydramine in urine and blood. There is some cross - reactivity detected with several tricyclic antidepressants, as well as cyclobenzaprine. This method can be applied to the detection of DPH in the toxicology field as part of a DUID panel.
References
1. Antihistamines and driving ability: evidence from on-the-road driving studies during normal traffic, Verster J.C., Volkerts E.R. Ann. Allergy Asthma Immunol. 2004, 92, 294-303.
2. Fatality from diphenhydramine monointoxication: a case report and review of the infant, pediatric and adult literature, Kuffner E., Patel M. Am. J. For. Med. Path. 2010, 31, 106
To view the full study and its data, visit this link.
Looking for a unit for your ELISA technique and other related applications? Check out our featured products below.