Daniel Soffer, William W. O'Neill, Kishore J. Harjai, Simon R. Dixon, Judith Boura, Robert D. Safian, Cindy L. Grines, Issam Moussa, Gary S. Roubin, Jeffrey W. Moses
William Beaumont Hospital, Royal Oak, Michigan, Lenox Hill Heart and Vascular Institute, New York, New York.
Background:
The degree of platelet inhibition (PI) induced by GPIIb/IIIa antagonists has been shown to influence clinical outcomes following percutaneous coronary intervention (PCI). There is no comparative data on the degree of PI using different commercially available point-of-care PI assays.
Methods:
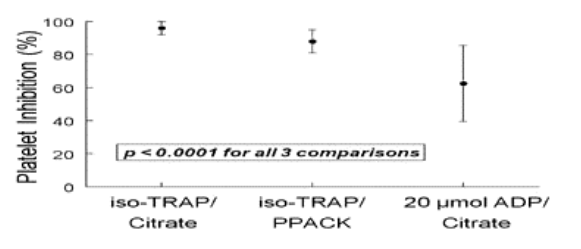
We prospectively enrolled 24 pts (66 ± 10 yrs, 18 males) who received a GPIIb/IIIa inhibitor during PCI. Pts received tirofiban; n=15 (10mcg/kg, 0.15mcg/kg/min), eptifibatide; n=7 (single bolus; 180mcg/kg, 2mcg/kg/min), and abciximab; n=2 (0.25mg/kg, 0.125mcg/kg/min). We compared the degree of PI using 3 different assays: 1) 20µmol ADP/citrate in the IchorTM platelet analyzer (Helena Laboratories, Beaumont, TX), 2) iso-TRAP/citrate and 3) iso-TRAP/PPACK as platelet agonists/anticoagulants respectively in the UltegraTM system (Accumetrics, San-Diego, CA). PI was measured in all pts 30 min following GPIIb/IIIa bolus, with each assay performed on the same blood sample.
Results:
The mean ± SD values of PI following GPIIb/IIIa administration are shown below.
Conclusion:
There is significant variation in the degree of PI assessed by the three assays. The greater inter-patient variability and the lower mean PI, detected by the IchorTM system may enhance patient stratification based upon response to GPIIb/IIIa inhibitors. The practical implications of these findings need to be validated in large-scale clinical outcome trials.
Looking for equipment or accessories from Helena Laboratories? Check out our featured products below.