Introduction:
> Antiplatelet therapy including aspirin (ASA) and clopidogrel is recognized as clinically important in patients at risk of developing thrombotic events
> Recently it has been realized that empiric therapeutic dosing is suboptimal due to inter-patient variability with regard to response, receptor concentration etc.
> Hence there is a clinical need to monitor such therapies on an individual basis
> Traditional platelet tests including light transmission aggregometry (LTA) are inconvenient for acute diagnostic testing.
> Hence, the introduction of "near-patient" test systems
> Here we describe the utility of a point of-care (POC) test platform (PlateletWorks®) for monitoring platelet response to ADP and arachidonic acid (AA) as the agonists
Methods & Procedure:
PlateletWorks (Helena Laboratories, Beaumont, TX) evaluates non-anticoagulated whole blood as the sample medium. It utilizes the principle of differential platelet counting in either the absence or presence of a platelet agonist (ADP, collagen, AA etc.) as a direct indication of platelet function. Results may be expressed as either % aggregation or %inhibition. In human clinical trials subjects (n=205) were evaluated for their response to ADP (20μM) and a second group of subjects (n=40) were evaluated for their response to AA (either 5μM or 1.25mg) on both the Plateletworks and LTA (Chronolog, Haverstown, PA) test platforms.
Results:
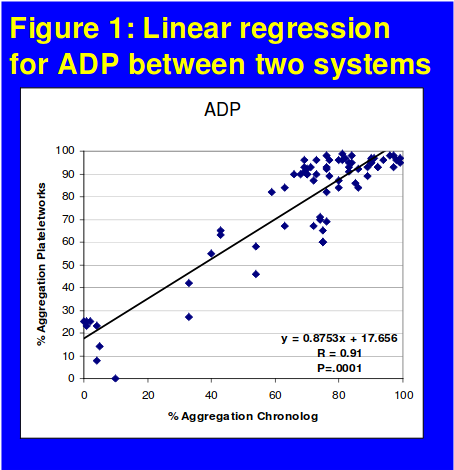
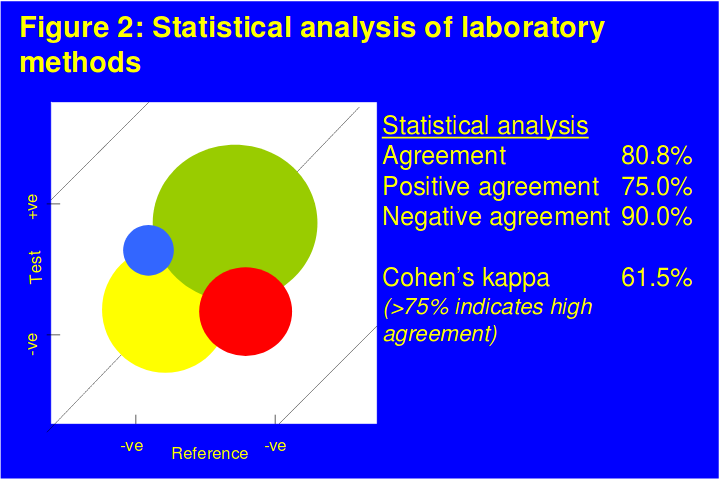
The comparative data for each agonist between the two platforms are shown in Figures 1 & 2. The correlation between the two systems for ADP was r=0.91. For AA, as LTA provides a qualitative and Plateletworks a quantitative measure of response data were analyzed using McNemar test of symmetry with a calculated Cohen's kappa value of 61.5%.
Conclusions:
> This novel POC test for platelet response maybe a suitable peri-procedural screening assay to determine the effective dose of administered pharmaceutical on an individual patient basis
> It may also provide clinical decision as to alternative antiplatelet therapies
> Large scale studies both in vascular disease and other disease processes are ongoing
Agonists: ADP-FDA approved ('91); AA - currently under FDA review
Looking for point-of-care monitoring and other related applications? Browse through our featured products below.