Kim Huynh, Michael Vincent ,Guohong Wang, Rehka Barhate, Warren Rodrigues, Christine Moore, James Soares
Immunalysis Corporation, Pomona, CA USA
Abstract
Methadone is metabolized by N-demethylation and cyclization to EDDP and further N-demethylation to EMDP. Due to variations in enzyme activity (CYP450 3A4) among individuals there are considerable variations in methadone metabolism and excretion (1- 3). Current immunoassays employed for screening methadone or EDDP in urine are extremely specific, resulting in some laboratories having to perform two immunoassay screens: one for methadone at a cut-off concentration of 300 ng/mL; one for EDDP at a cut-off concentration of 100 ng/mL.
Objectives
Our objective was to develop a single homogeneous immunoassay (HEIA) with significant cross reactivity to methadone and its metabolites. Such an assay would enable laboratories to screen urine for methadone and minimize the reporting of false negatives containing EDDP only due to fast metabolization.
Advantages
- Assaying for both methadone and EDDP instead of methadone could be useful in determining compliance among "fast metabolizers" (EDDP but no methadone).
- Identifying urines that contain methadone only "poor metabolizers" or could be adulterated ("Spike") that could not be detected by EDDP assay
- Eliminating false negative results caused by using methadone or EDDP assay only
- Suitable for oral fluid methadone testing
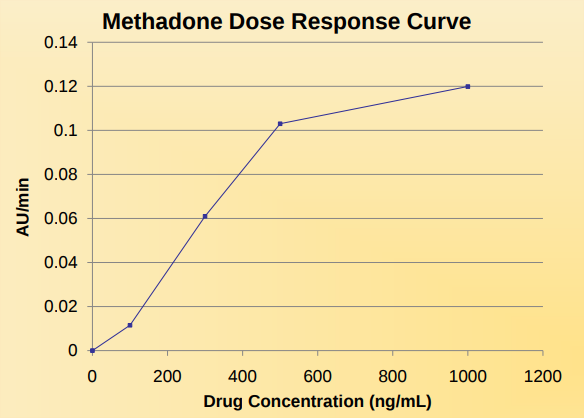
Results and Discussion
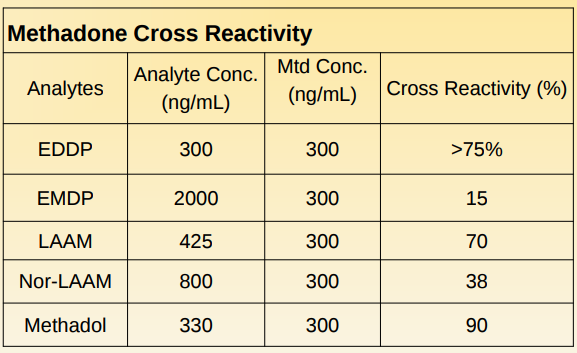
Cross Reactivity:
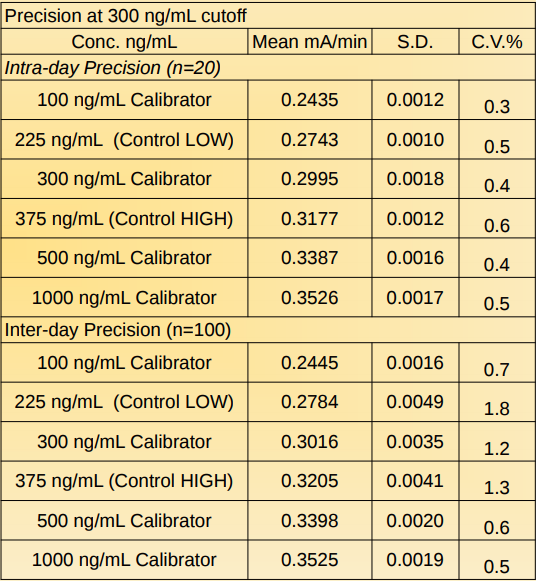
Precision: Daily Calibration Required
Precision was determined by testing calibrators and controls for 5 days, 5 runs per day in replicates of 4 (N=100).
Authentic specimens:
244 urine specimens were obtained from commercial laboratories. The specimens were a combination of positive and negative urines, and were analyzed by the HEIA and by LC-MS/MS using cutoff s of 300ng/mL HEIA, and methadone and EDDP at 100 ng/mL for LCMS.
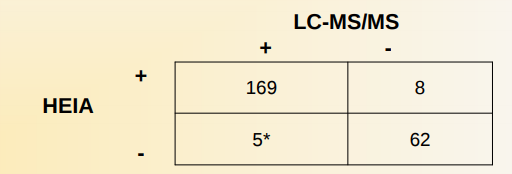
The sensitivity, specificity, and accuracy were calculated to be 97%, 89%, and 95%, respectively.
®Screening/LCMS(methadone/EDDP):172/(184/107); 213/(0/139);267/(142/240); 225/(0/150);224/(0/215).
Summary
An improved methadone and EDDP HEIA has been developed and should be useful for eliminating the false negative results caused by methadone assay only.
References
- R.A. Totah,K.E.Allen, P.Sheffels,D.Whittington, E.D. Kharasch. Enantiontiomeric metabolic interactions and steroselective human methadone metabolism. J. Pharmacol. Exp. Ther.321:389-399(2007).
- P.J. Orsulak, L.C. Akers and N. Schuyler. Clinical application of the CEDA EDDP (methadone metabolite) assay. Poster section 2, SOFT-TIAFT 1998.
- K.L.Preston, D.H. Epstein, D.Davoudzadeh and M.A. Huestis. Methadone and metabolite urine concentration in patients maintained on methadone. J. Anal. Toxicol. 27:332-341(2003).
SOFT, Richmond, VA 2010
To view the full study and its data, visit this link.
Looking for equipment or accessories for your immunoassay and other related applications? Check out our products available on Block Scientific Store today!