For Business Use Only. Does Not Ship to Residential Addresses. For use inside an Analyzer, Sold Separately.
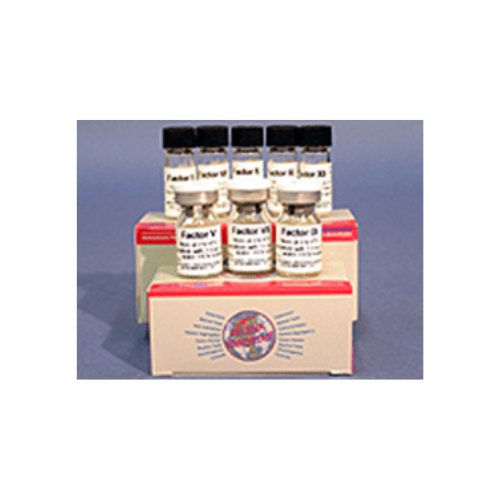
Helena Factor IX (10x1mL)
Product Code: 5194
Manufacturer: Helena Laboratories
Shipping Weight: 5.00lbs (2.27kg)
Factor IX (10x1mL)
Factor IX Deficient Substrate, 10 x 1 mL
Intended Use
The Factor IX Deficient Substrate Plasma is intended for the quantitative determination of Factor IX (Christmas factor) in patients suspected of having a congenital or acquired deficiency of this coagulation protein.
Summary
Numerous coagulation factors have been identified in the blood and are required for normal blood clotting. A deficiency of one or more of the factors may result in a notable hemorrhagic condition, the severity of which is governed by the degree of the deficiency. Deficiencies of the blood clotting factors may be congenital or acquired. The congenital deficiencies are, in general, single deficiency states while the acquired deficiencies may be multiple in nature and commonly associated with liver disease, vitamin K deficiency or the ingestion of coumarin type anticoagulant drugs, and defibrination secondary to intravascular clotting.
Factor IX, known as Christmas Factor or plasma thromboplastin component (PTC), is decreased in a congenital disease known as Hemophilia B or Christmas Disease. Hemophilia B is clinically indistinguishable from hemophilia A, and it has a sex-linked recessive mode of inheritance. There are about 7 cases of hemophilia A to every case of hemophilia B.
An acquired Factor IX deficiency may occur in conjunction with a vitamin K deficiency and/or hepatocellular dysfunction.
In an effort to devise a quantitative assay for Factor IX, several methods based on the thromboplastin test were used and were found to be time consuming and complicated. Langdell, Wagner and Brinkhous (1953) developed a one-stage “partial thromboplastin time” which was simple to perform but not reproducible. Helena’s procedure determines Factor IX activity by using a modification of the activated partial thromboplastin time (APTT) test and a Factor IX deficient substrate plasma.