Cynthia Coulter, Margaux Garnier, James Tuyay, Christine Moore,
Immunalysis Corporation, Pomona, CA, U.S.A.
Background
➢ An investigation into the stability of drugs in oral fluid using the Quantisal® collection device during normal storage and transportation conditions was carried out.
➢ The objective was to determine optimal conditions for sample handling for both collector and laboratory.
➢ Three separate experiments were conducted
➢ stability at room temperature (25ºC)
➢ stability at refrigerated temperature (4ºC)
➢ stability during overnight transportation
Method
➢ Drug concentration was determined by:
➢ LC-MS/MS for amphetamine (AMP), methamphetamine (METH), benzoylecgonine (BZE), phencyclidine (PCP), morphine (MOR), and oxycodone (OXYC)
➢ GC-MS for ∆9-tetrahydrocannabinol (THC)
➢ All methods are fully validated and tested against both proficiency and real user specimens.
Experiment
➢ 50mL of neat oral fluid was collected, pooled, and divided into two 25mL aliquots
➢ Multi-analyte drug solutions were prepared at:
➢ -50% (solution A) and +50% (solution B) of the cutoff
➢ Cutoff concentrations:
➢AMP (50ng/mL), METH (50ng/mL), BZE (15ng/mL), PCP (10ng/mL)
➢MOR (30ng/mL), OXYC (30ng/mL), THC (4ng/mL)
➢ 16 collection pads soaked up 1mL of fortified oral fluid for each level
➢ When the collection pad indicator turned blue each pad was placed into a corresponding labeled tube
➢ Samples were stored at room temperature in the dark and analyzed in duplicate at time points 0, 7, 14, and 30 days
Samples were stored at 4°C and analyzed in duplicate at time points 14 and 30 days
➢ After analysis at time zero, specimens were packed in a thermo container which included temperature and humidity logs; shipped overnight to the East coast and back for analysis
Results
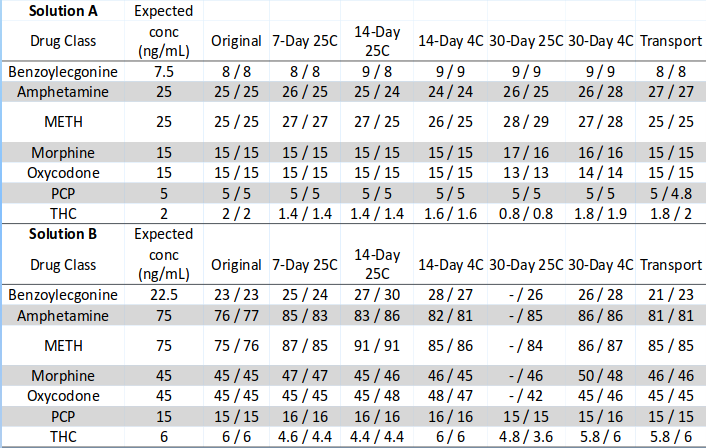
➢ An analytical result within +-20% of the original value (day zero) was considered to be an acceptable analytical variable
➢ All drugs except THC were within this range up to day 30 when stored at room temperature in the dark
➢THC showed a 30% and 25% loss for solutions A and B respectively by day 7 with a 60% and 30% loss by day 30
➢ At refrigerated temperature (4°C) all drugs at all times points for both solutions A and B showed minor degradation and were within +-20% range
➢THC showed a 10% and 2% loss for solutions A and B by day 30 when stored at refrigerated temperature
➢ The temperature during overnight transportation ranged from 14.8 - 30.1°C; no significant loss was seen for any drug
➢ The low concentration THC solution had a minor loss of 10% while the +50% solution had a loss of 3%
Data
Conclusion
➢ Drugs are stable within the Quantisal® oral fluid collection device when shipped overnight without cold packs in regulation containers.
➢ Once received into the laboratory the Quantisal® device should be stored refrigerated at 4°C especially if THC analysis is required.
➢ Samples should be analyzed within the first week of receiving but can be stored for at least 30 days without significant loss. This has proven to be helpful for re-analysis purposes.
SOFT, Orlando, 2013
To view the full data, click here.
Check out these reagent products from Immunalysis on Block Scientific Store: